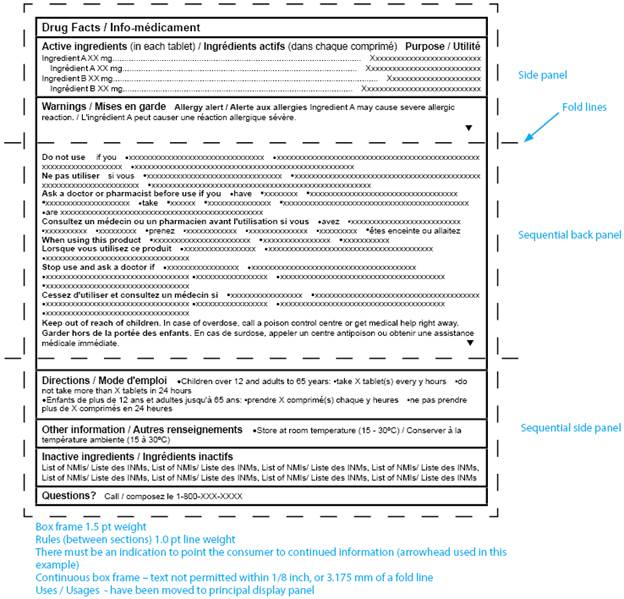
Prescription Label Requirements Canada . the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. this document provides information for industry on how health canada's health products and food. this document provides information for industry on how health canada’s health. The recommendations provided in this guide. The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable to inner and. it is essential that all labelling and packaging regulatory requirements be met.
from www.canada.ca
health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. this document provides information for industry on how health canada’s health. this document provides information for industry on how health canada's health products and food. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. it is essential that all labelling and packaging regulatory requirements be met. it is essential that all labelling and packaging regulatory requirements be met. The recommendations provided in this guide. The recommendations provided in this guide. • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable to inner and.
Labelling requirements for nonprescription drugs guidance document
Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. it is essential that all labelling and packaging regulatory requirements be met. it is essential that all labelling and packaging regulatory requirements be met. The recommendations provided in this guide. The recommendations provided in this guide. this document provides information for industry on how health canada's health products and food. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. this document provides information for industry on how health canada’s health. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable to inner and.
From www.youtube.com
Food Labeling Requirements Health Canada Front of Package (FOP Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. it is essential that all labelling and packaging regulatory requirements be met. The recommendations provided in this guide. this document provides information for industry on. Prescription Label Requirements Canada.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Prescription Label Requirements Canada health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. it is essential that all labelling and packaging regulatory requirements be met. this document provides information for industry on how health canada's health products and food. The recommendations provided in this guide. it is essential that. Prescription Label Requirements Canada.
From astronovaproductid.com
New Labeling Regulations for Canadian Natural Health Products Prescription Label Requirements Canada this document provides information for industry on how health canada’s health. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. this document provides information for industry. Prescription Label Requirements Canada.
From www.canada.ca
Labelling requirements for nonprescription drugs guidance document Prescription Label Requirements Canada this document provides information for industry on how health canada’s health. it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. The recommendations provided in this guide. it is essential that all labelling and. Prescription Label Requirements Canada.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Prescription Label Requirements Canada this document provides information for industry on how health canada’s health. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. The recommendations provided in this guide. this document provides information for industry on how health canada's health products and food. it is essential that all. Prescription Label Requirements Canada.
From exoduzryz.blob.core.windows.net
Fda Label Guidance at Debra Jumper blog Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. this document provides information for industry on how health canada’s health. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. the objective of the good label and package practices guide for prescription drugs. Prescription Label Requirements Canada.
From exojlwvqr.blob.core.windows.net
What Is A Labeling Requirement For Food Products In Canada at Sally Prescription Label Requirements Canada the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for.. Prescription Label Requirements Canada.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. The recommendations provided in this guide. The recommendations provided in this guide. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable. Prescription Label Requirements Canada.
From www.futures-supplies.co.uk
Regulations News Flash Prescription Label Requirements Canada The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. The recommendations provided in this guide. . Prescription Label Requirements Canada.
From animalia-life.club
Fda Drug Labeling Requirements Prescription Label Requirements Canada • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable to inner and. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. it is essential that all labelling and packaging regulatory requirements be met. this document provides information for industry on. Prescription Label Requirements Canada.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. this document provides information for industry on how health canada's health products and food. it is essential that all labelling and packaging regulatory requirements. Prescription Label Requirements Canada.
From ar.inspiredpencil.com
Prescription Medicine Label Prescription Label Requirements Canada health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,.. Prescription Label Requirements Canada.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. it is essential that all. Prescription Label Requirements Canada.
From www.heb.com
HEB Pharmacy Taking Care Of Texans Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. this document provides information for industry on how health canada's health products and food. the objective of the good label and package practices guide. Prescription Label Requirements Canada.
From www.youtube.com
How to read a medication label YouTube Prescription Label Requirements Canada this document provides information for industry on how health canada's health products and food. The recommendations provided in this guide. this document provides information for industry on how health canada’s health. health canada and ismp canada are pleased to announce the release of the good label and package practices guides for. The recommendations provided in this guide.. Prescription Label Requirements Canada.
From www.slideserve.com
PPT VETERINARY DRUG USE AND PRESCRIBING Chapter 5 PowerPoint Prescription Label Requirements Canada The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. this document provides information for industry on how health canada’s health. this document provides information for industry on how health canada's health products and food. it is essential that all labelling and packaging regulatory requirements be met. The. Prescription Label Requirements Canada.
From ctocrx.com
Fill Prescription Now Coast to Coast Compounding Prescription Label Requirements Canada The recommendations provided in this guide. it is essential that all labelling and packaging regulatory requirements be met. • applicable canadian regulations, standards, policies, and guidelines • health canada risk communications applicable to inner and. this document provides information for industry on how health canada's health products and food. health canada and ismp canada are pleased. Prescription Label Requirements Canada.
From www.etsy.com
Prescription Label Template Editable Horizontal RX Bottle Etsy Canada Prescription Label Requirements Canada it is essential that all labelling and packaging regulatory requirements be met. The recommendations provided in this guide. The recommendations provided in this guide. the objective of the good label and package practices guide for prescription drugs is to provide direction to sponsors,. health canada and ismp canada are pleased to announce the release of the good. Prescription Label Requirements Canada.